Testing is an important weapon in every hemp producer’s arsenal when defending against microbial contamination. The following are Botanacor’s three new microbial tests:
- Pseudomonas aeruginosa
- Staphylococcus aureus
- Listeria monocytogenes
Here is more information on the microorganisms and why you should consider these tests before launching your next product.
Staphylococcus aureus
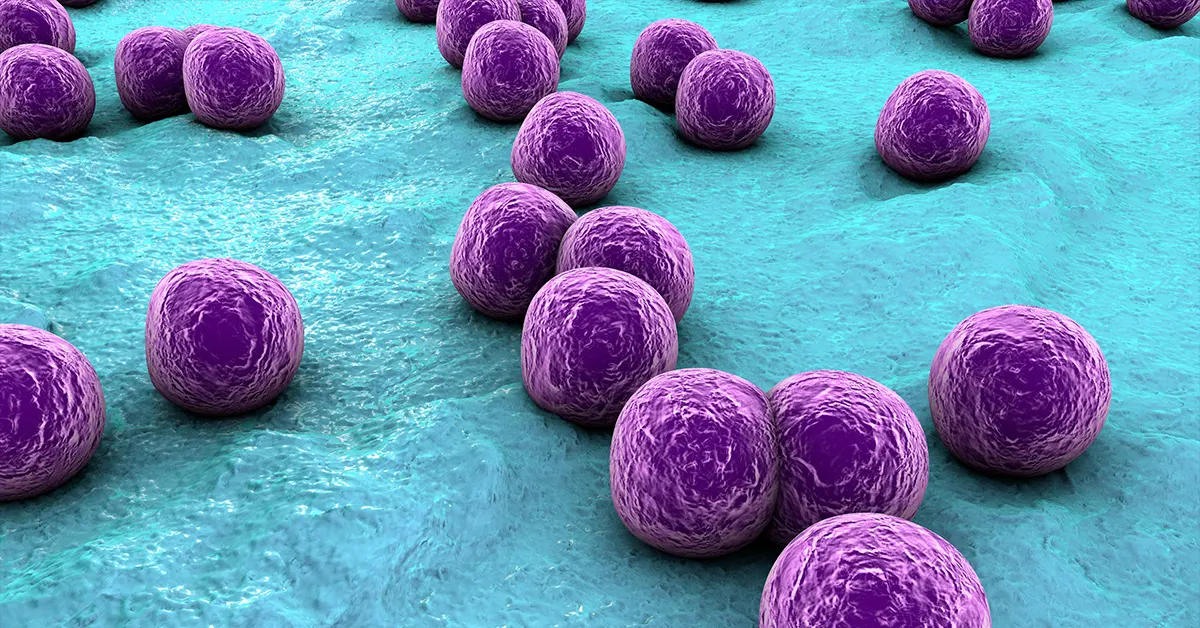
Staphylococcus aureus
What is it?
Staphylococcus aureus is a gram-positive bacteria that causes infectious diseases such as skin infections, pneumonia and food poisoning[1]:
- Gram-positive bacteria
- Staphylococcus aureus is present in the nose of about 30% of healthy adults and on the skin of about 20% of people.
- In Latin staphyle means “a bunch of grapes”; aureus means “gold”
- Microscopically appears as clusters of round cells, resembling grapes after gram-staining
- Appears as yellow colonies when grown on specific nutrient media
What are the health implications?
- One of the most common causes of hospital-acquired infections
- One of the five most common causes of infection after injury or surgery
- Certain strains of Staphylococcus aureus can be antibiotic resistant
Recalls
- https://www.foodsafetynews.com/2015/07/test-results-show-philippines-outbreak-caused-by-staphylococcus/
- https://www.illinoisinjurylawyerblog.com/face_lotion_recalled_because_o_1/
Pseudomonas aeruginosa
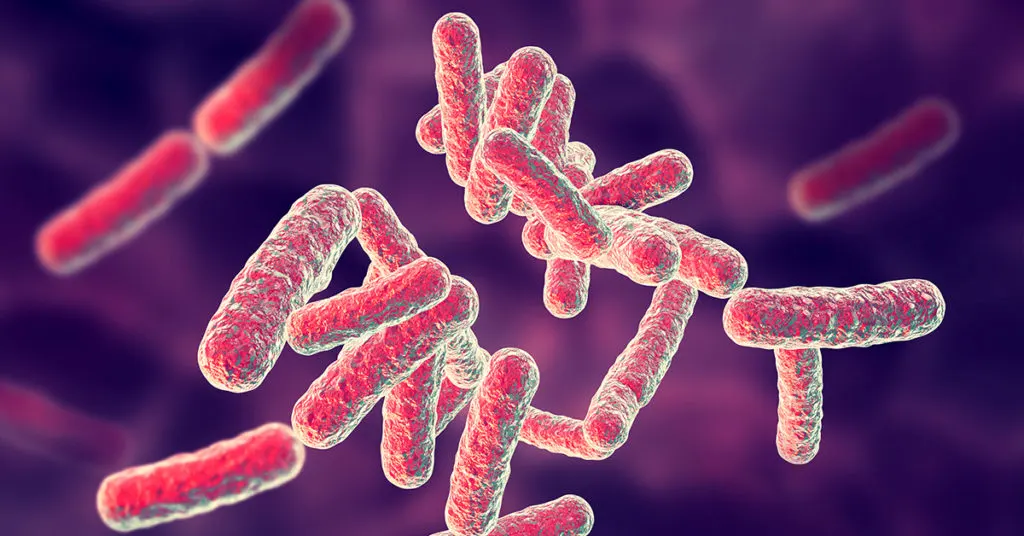
Pseudomonas aeruginosa
What is it?
- Gram-negative bacteria[2]
- Found commonly in water and soil and can grow on food
- In Latin, aeruginosa means “copper rust”
- Appears as blue-green when grown on specific nutrient media
What are the health implications?
- One of the most common causes of nosocomial infections
- Strongly adaptable, resistant to many disinfectants and antimicrobial agents
- Can cause pneumonia, UTIs, GI infections and more
- Much more of a health risk for immunocompromised people/animals
Recalls
- https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/out-abundance-caution-maryruths-announces-voluntary-recall-two-lots-its-liquid-probiotic-infants#:~:text=MaryRuth’s%2C%20a%20leading%20omni%2Dchannel,possibility%20of%20contamination%20by%20Pseudomonas
- https://pubmed.ncbi.nlm.nih.gov/10945589/
Listeria monocytogenes
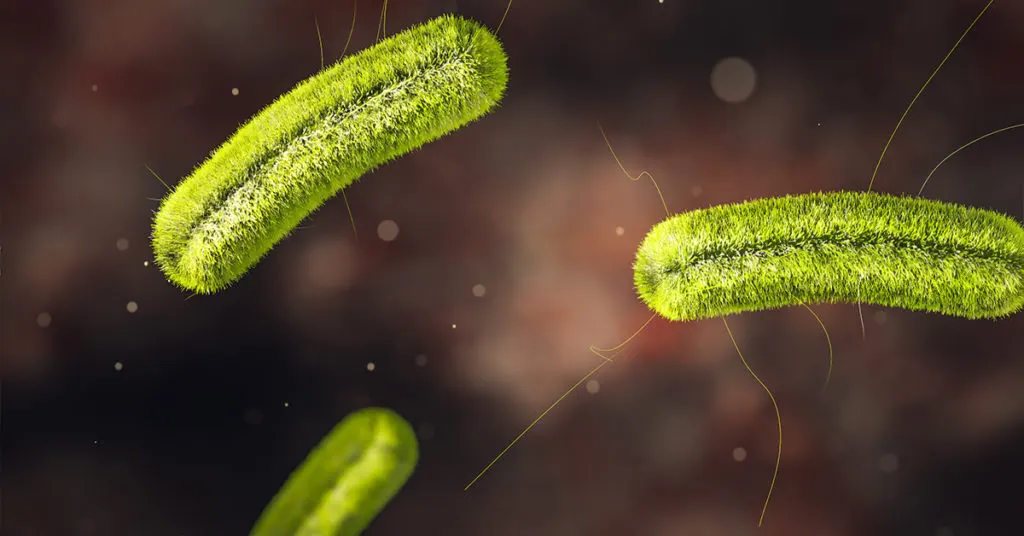
Listeria monocytogenes
What is it?
- Gram-positive bacteria
- Found in moist environments, soil, water, decaying vegetation, and can survive under many food preservation measures[3]
What are the health implications?
- When ingested, causes listeriosis: fever, aches, nausea, vomiting, diarrhea. Severe infections can result in confusion, loss of balance, convulsions and even death
- Top five bacteria causing “food poisoning”; cause of the most food sickness-related recalls
- Particularly dangerous for pregnant women
- Unlike most bacteria, it can continue to grow at refrigerated temperatures
Recalls
How are we testing?
We test both Staphylococcus aureus and Pseudomonas aeruginosa following USP <62> (united states pharmacopeia) procedure[4], which is used as a reference method for non-sterile pharmaceutical product testing. Validated in-house for use in cannabis-infused matrices. Species level confirmation testing through analytical profile index testing, which allows for the classification of bacteria based on a series of biochemical tests.
We test Listeria monocytogenes following an FDA recommended method that is used for food and environmental testing[5]. Validated in-house for use in cannabis-infused matrices. Species level confirmation testing through analytical profile index testing, which allows for the classification of bacteria based on a series of biochemical tests.
Finally, to recap—why should you test your hemp products for microbial contamination?
- Avoid costly lawsuits
- Protect and enhance brand image
- Stay compliant with regulations
Coming soon—we are adding Aspergillus to our microbial panel in early 2022! Stay tuned for more information!
References
- [1] Gnanamani, et al., “Staphylococcus aureus: Overview of Bacteriology, Clinical Diseases, Epidemiology, Antibiotic Resistance and Therapeutic Approach”, InTechOpen, accessed December 27, 2021, https://www.intechopen.com/chapters/54154
- [2] Moradali et al., “Pseudomonas aeruginosa Lifestyle: A Paradigm for Adaptation, Survival, and Persistence”, Frontiers in Cellular and Infection Microbiology, accessed December 27, 2021, https://www.frontiersin.org/articles/10.3389/fcimb.2017.00039/full
- [3] “Listeria (Listeriosis)”, FDA, accessed December 27, 2021, https://www.fda.gov/food/foodborne-pathogens/listeria-listeriosis
- [4] US Pharmacopeia, “Microbiological Examination of Nonsterile Products: Tests for Specified Microorganisms”, accessed December 27, 2021, https://www.usp.org/sites/default/files/usp/document/harmonization/gen-method/q05a_pf_ira_34_6_2008.pdf
- [5] Hitchins et al., “BAM Chapter 10: Detection of Listeria monocytogenes in Foods and Environmental Samples, and Enumeration of Listeria monocytogenes in Foods”, accessed December 27, 2021, https://www.fda.gov/food/laboratory-methods-food/bam-chapter-10-detection-listeria-monocytogenes-foods-and-environmental-samples-and-enumeration
