Table of Contents
Introduction
Background
Chromatography 101
Case Study: Delta-8 THC Sample
Conclusion
In 2020, the hemp industry saw the rise in popularity of hemp-derived Delta-8 THC. From vape cartridges to infused products, Delta-8 THC has become the hot new cannabinoid in the hemp marketplace.
After performing cannabinoid potency analysis, some customers call us, concerned that we found both Delta-8 and Delta-9 cannabinoids in their sample.
This simply isn’t a fun discussion to have on the phone, for either of us. This short article illustrates the differences between the two cannabinoids and how the use of chromatography enables us to completely separate and quantify them.
If the idea of reading about Chemistry seems painful, our companion article discusses the same topic for the layman.
Just looking for the chromatograms and UV visualization? [Jump down to here.]
What is the difference between Delta-9 and Delta-8 THC?
Delta-9 and Delta-8 THC are isomers, which means that they have identical molecular formulas, or the same number of atoms, but the configuration of each are unique. In the image below, you can see the numbers on each of the atoms around the structure always beginning with the hydroxyl (OH) attachment position. There is only one very subtle difference between these two isomers and it has to do with the placement of the double bond (two lines) that occur either between the 9-10 position or the 8-9 position.
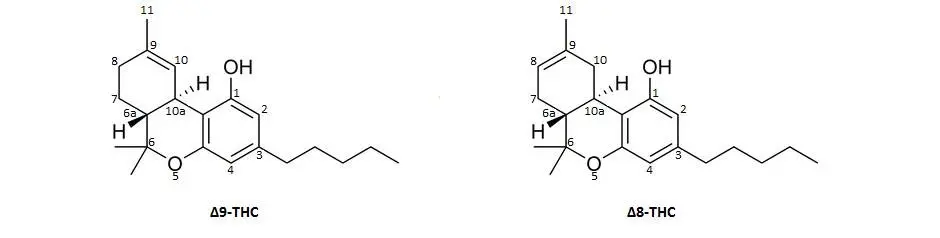
How is Delta-8 THC made?
The DEA released their Interim Final Rule, stating, “all synthetically derived tetrahydrocannabinols remain schedule I controlled substances.” Delta-8 is included on the DEA’s Controlled Substances list as well (see page 17).
Some legal opinions state that since Delta-8 THC comes from hemp, it does not fall under the DEA’s definition of “synthetic”. This argument will most likely go on for a while due to differences in language and jurisdiction between the USDA (Farm Bill) and the DEA Controlled Substances List.
Analytical testing laboratories use chromatography to determine the concentration of cannabinoids. By using analytical instruments such as High-Pressure Liquid Chromatography (HPLC), a laboratory can separate the cannabinoids by “retention time.” That time signature is then compared against a reference standard of a known concentration that has the same retention time. The data is then converted into a graphical representation called a chromatogram.
What is Chromatography?
Chromatography is a process for separating components of a mixture. To get the process started, the mixture is dissolved in a substance called the mobile phase, which carries it through a second substance called the stationary phase.
The different components of the mixture travel through the stationary phase at different speeds, causing them to separate from one another. The nature of the specific mobile and stationary phases determines which substances travel more quickly or slowly and is how they are separated. These different travel times are termed retention time.[1]
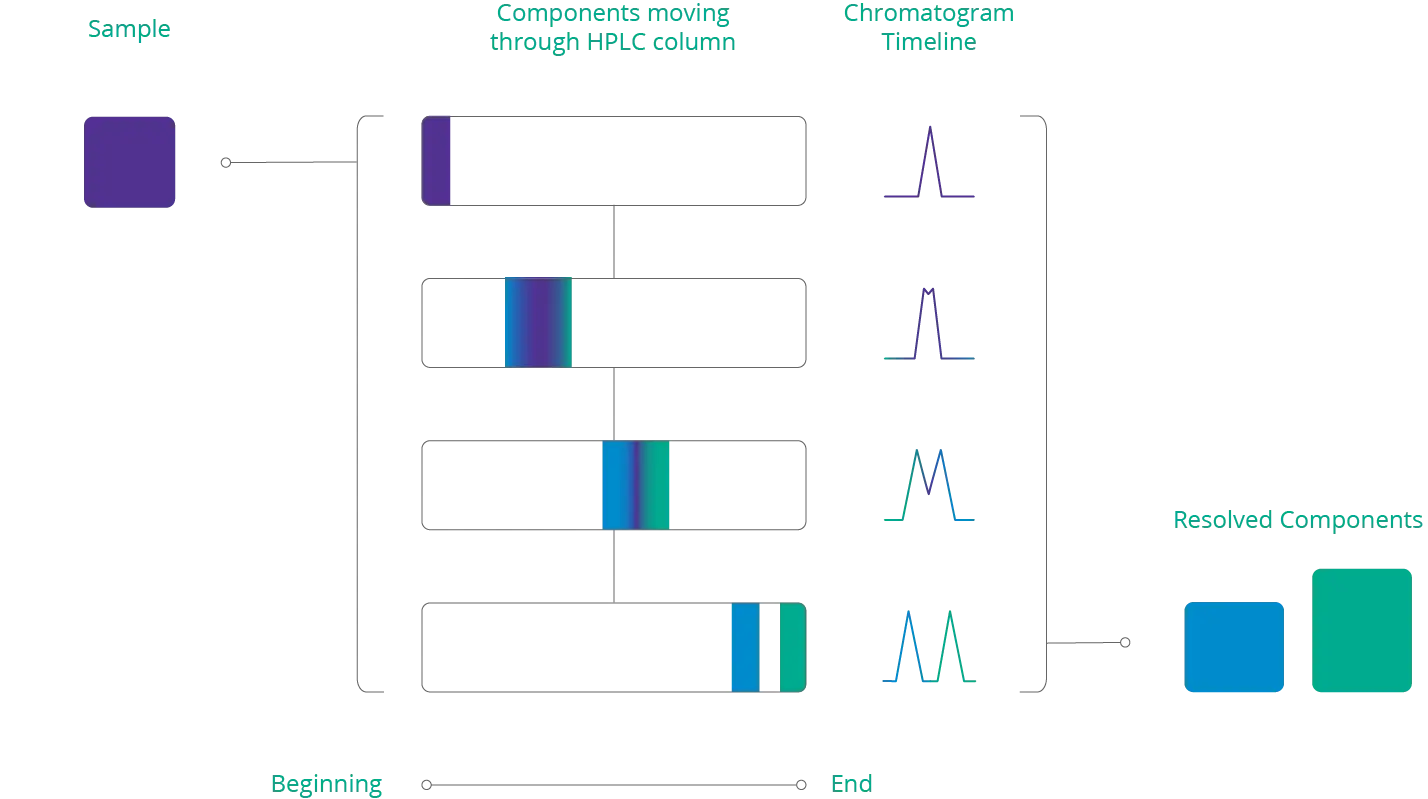
Below is an illustration of a sample that separates into different components as it travels through an HPLC column. When the sample exits the column, the cannabinoids can be identified by their retention time.
Resolution
To determine the concentration of a cannabinoid, laboratories need to differentiate between each cannabinoid that is tested. The area under each discrete peak is calculated and that relates directly to the amount of analyte on the instrument. If two cannabinoids are too close together, the laboratory cannot accurately determine the concentration of each cannabinoid due to the overlap.
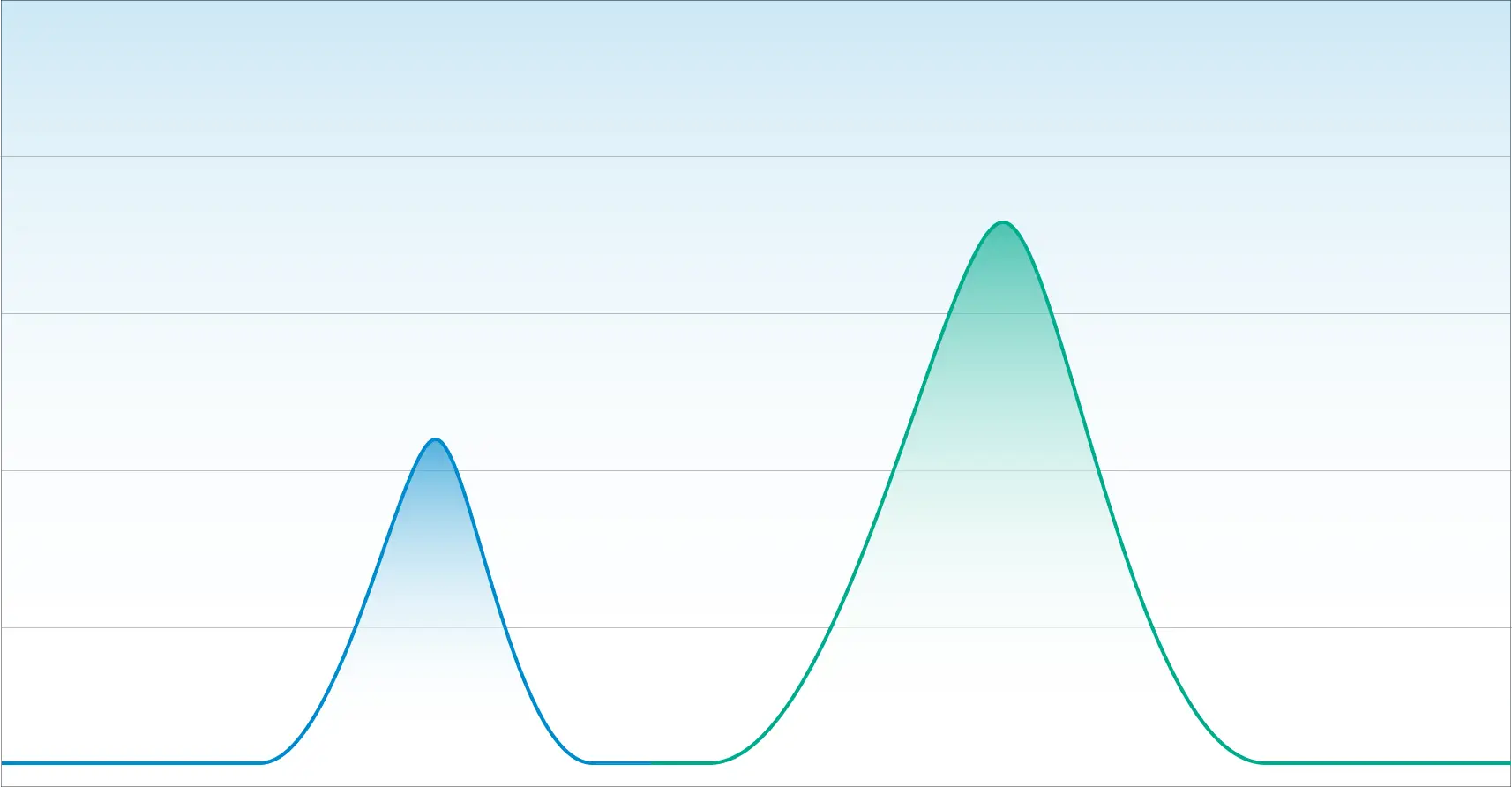
Below is a chromatogram that illustrates two components with good resolution. You can see the beginning and the end and at no point do the two peaks overlap.
In our next example, we have a chromatogram that illustrates poor separation. While this appears to look like one chromatographic peak, it is two overlapping peaks.
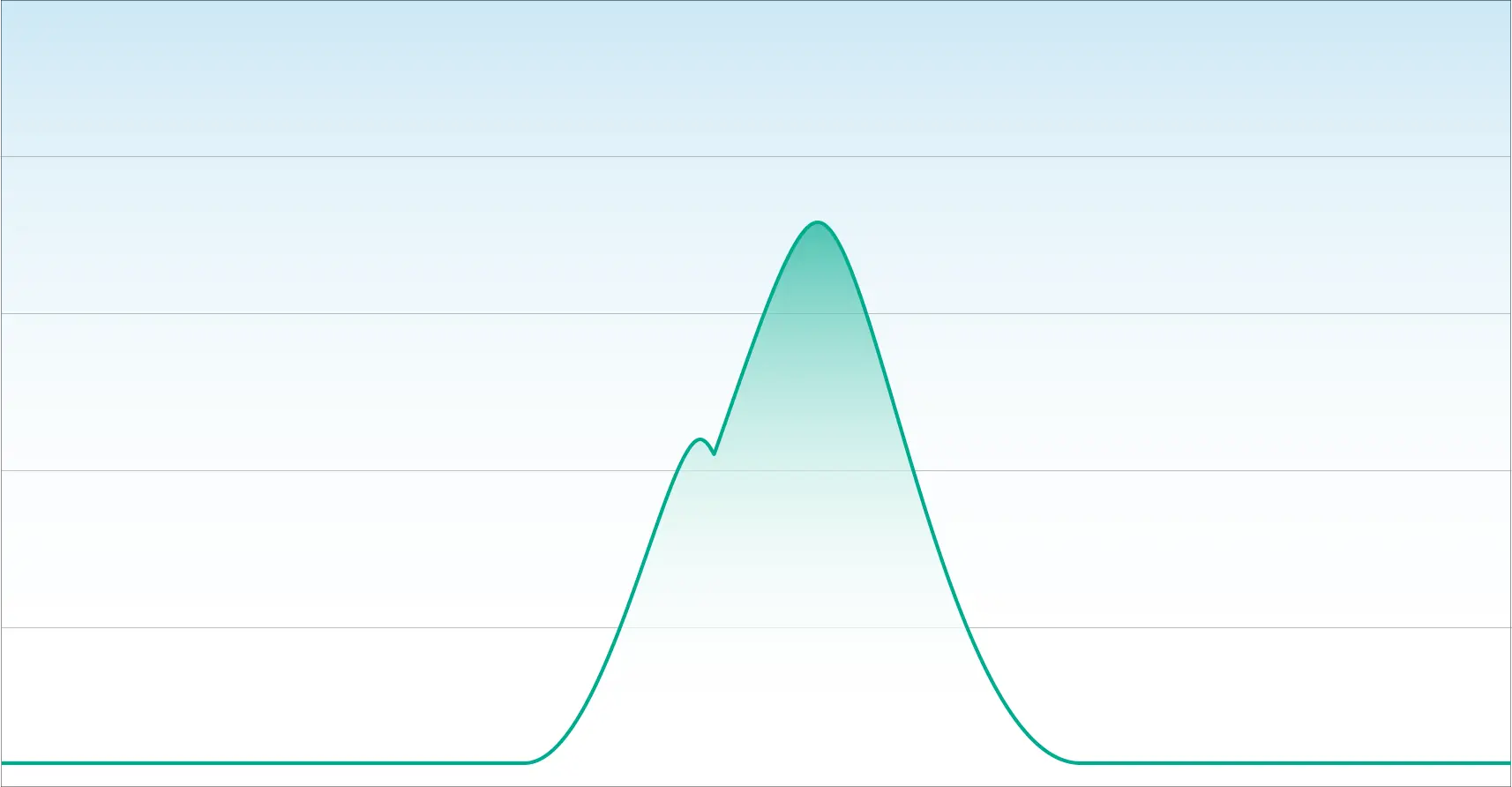
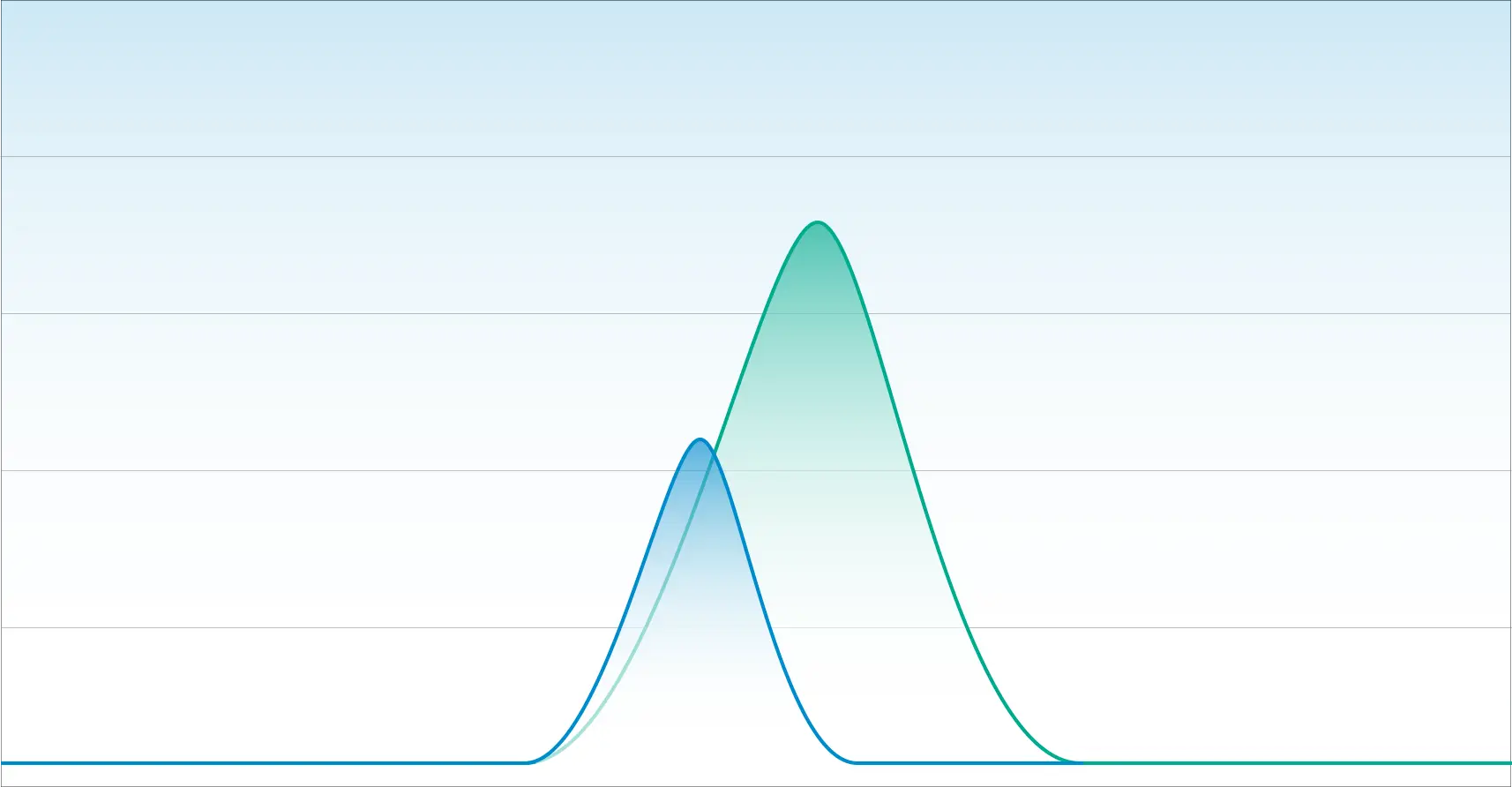
An inexperienced laboratory with a deficient method will quantitate this as one cannabinoid. This is what happens when laboratories cannot distinguish Delta-9 THC from Delta-8 THC.
Calculating Resolution
As cannabinoids pass through an HPLC column, there is a mathematical calculation to describe the actual separation of the peaks or “resolution” labeled Rs.
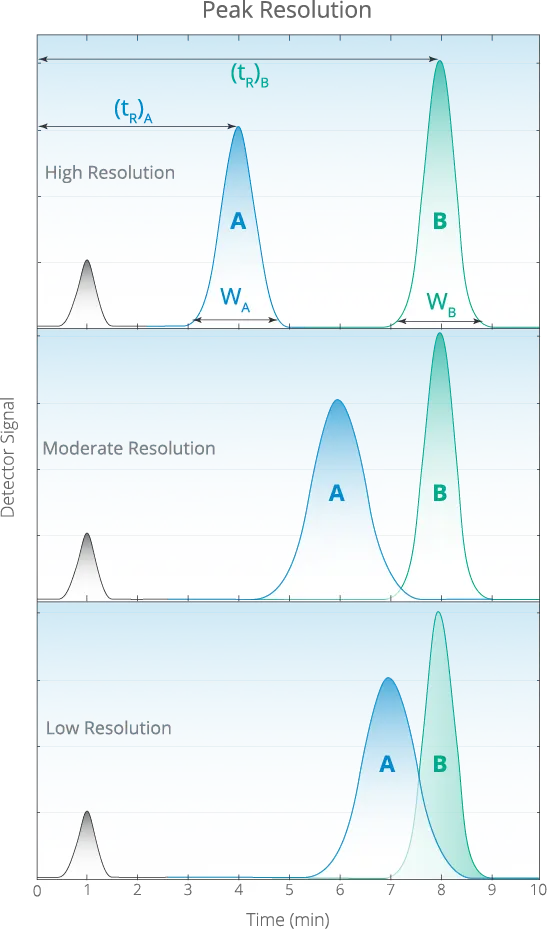
Resolution is calculated as follows:
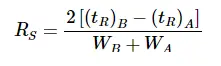
- High Resolution The greater the distance between the two chromatographic peaks, the higher the confidence that the chemist accurately separated the two components.
- Moderate Resolution The lesser the distance between the two chromatographic peaks, the confidence in the separation decreases.
- Low Resolution If the peaks overlap, they cannot be accurately integrated.
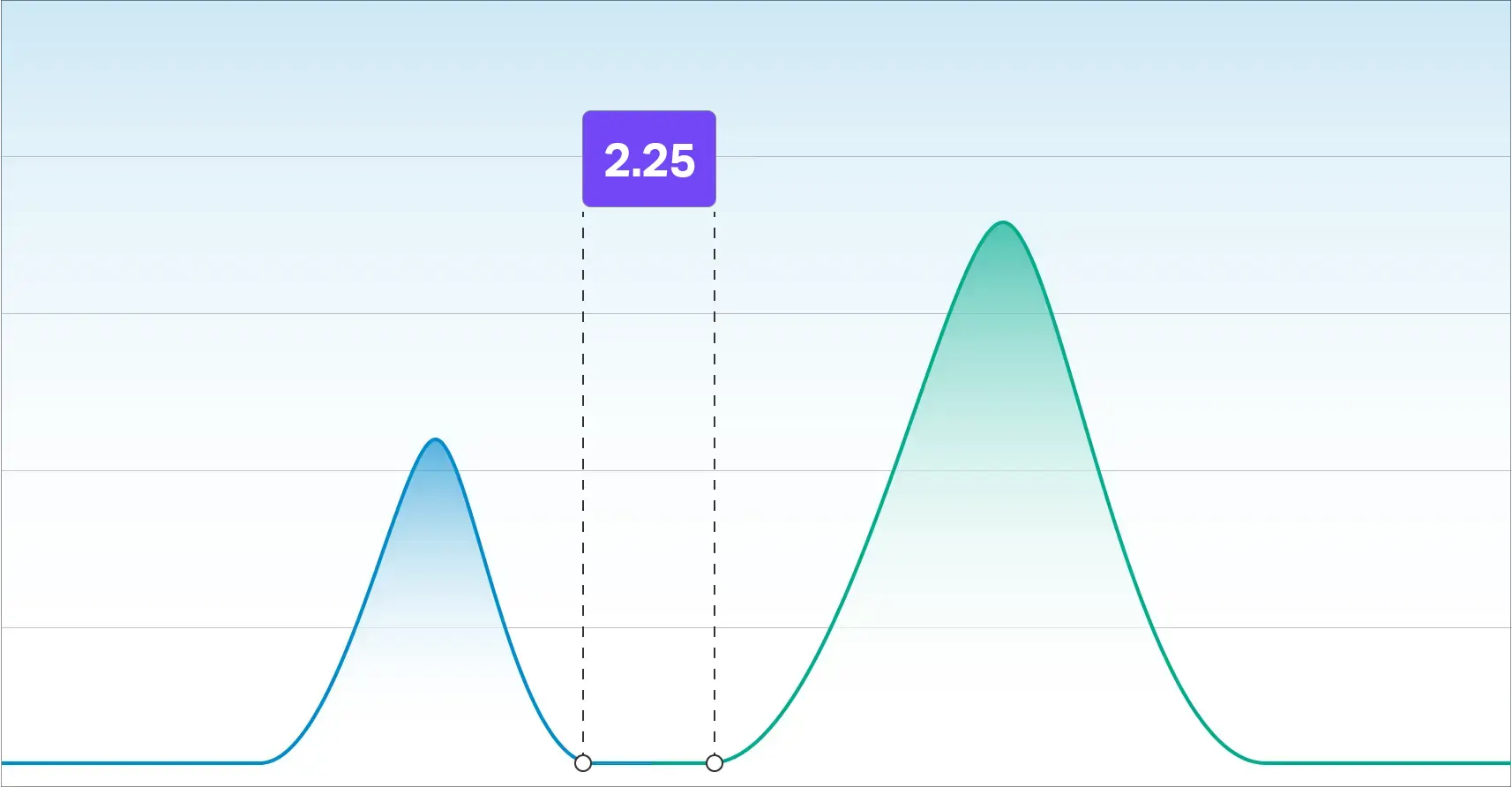
According to The US Pharmacopeia, a resolution factor of 1.5 is required for accurate integration. Our resolution factor between Delta-9 THC and Delta-8 THC is 2.25 which is well beyond the requirement for quantitating two species independently.
Reference Standards
Reference Standards are what laboratories use to identify cannabinoids in a sample. They are supplied by manufacturers that create pure cannabinoids of a known concentration.
To demonstrate our ability to separate Delta-9 THC from Delta-8 THC, we created three different concentrations (high, mid, and low) of our reference standards and ran them through our HPLC.
High Concentration Standards
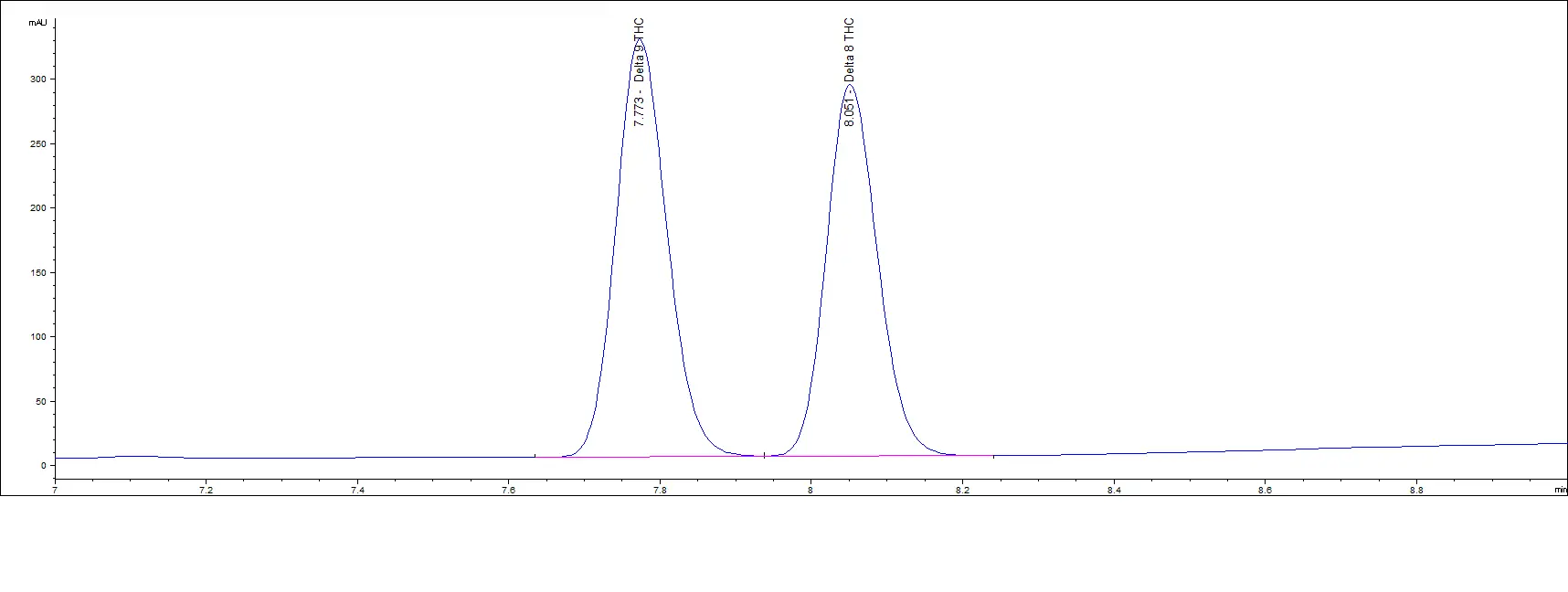
Mid Concentration Standards
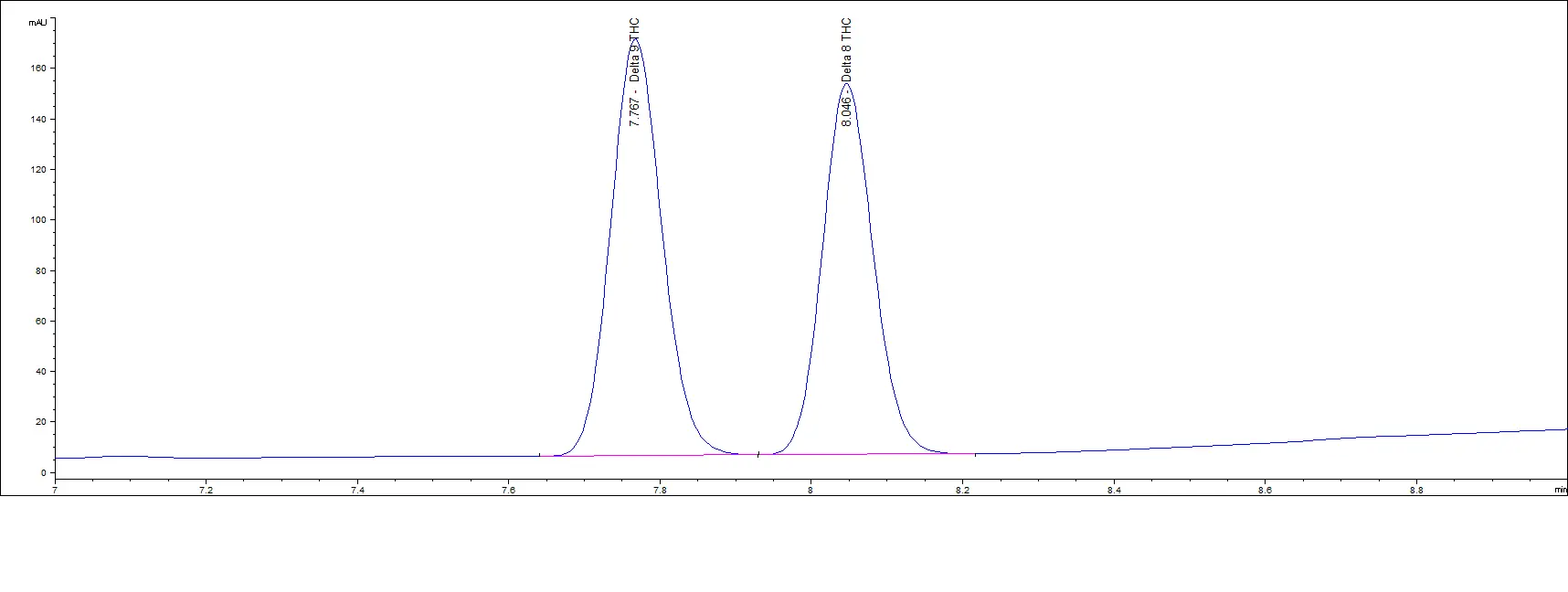
Low Concentration Standards
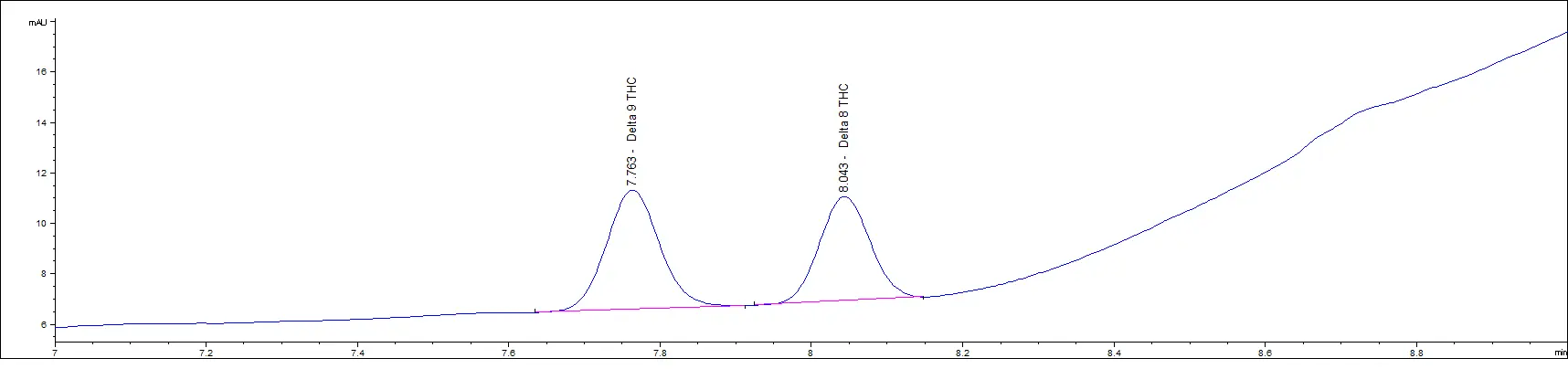
As you can see in the above chromatograms, no matter the concentration, the retention times are the same and we can easily separate each peak.
System Suitability
System suitability is an overlooked aspect in analytical hemp/cannabis testing. System suitability is used to prove that a system is working acceptably before analysis. In regulated industries that the FDA oversees, system suitability is a requirement. To date, system suitability is not required by any state authority governing hemp or cannabis testing, however we require it to ensure data defensibility.
As part of our daily system suitability check, we use our primary set of reference standards to create our calibration curve. We use a second set of reference standards, from a second qualified vendor, to compare against our primary set. This is called the check standard.
After every 10 injections, we run a check standard against the ten samples that we just tested. This process is repeated throughout the entire day. By running check standards, the lab can determine if there was an issue, it only affects those previous 10 samples, which would need to re-run.
We routinely test Delta-8 THC products in various matrices and have been testing these for years. Recently, we received a Delta-8 THC sample, and we reported the presence of Delta-9 THC. The customer was told their product did NOT have Delta-9 THC in the product.
Below is the customer’s chromatogram and you can see that we were able to easily separate the two cannabinoids. The peaks align with the retention times of our reference standards.
Customer Sample
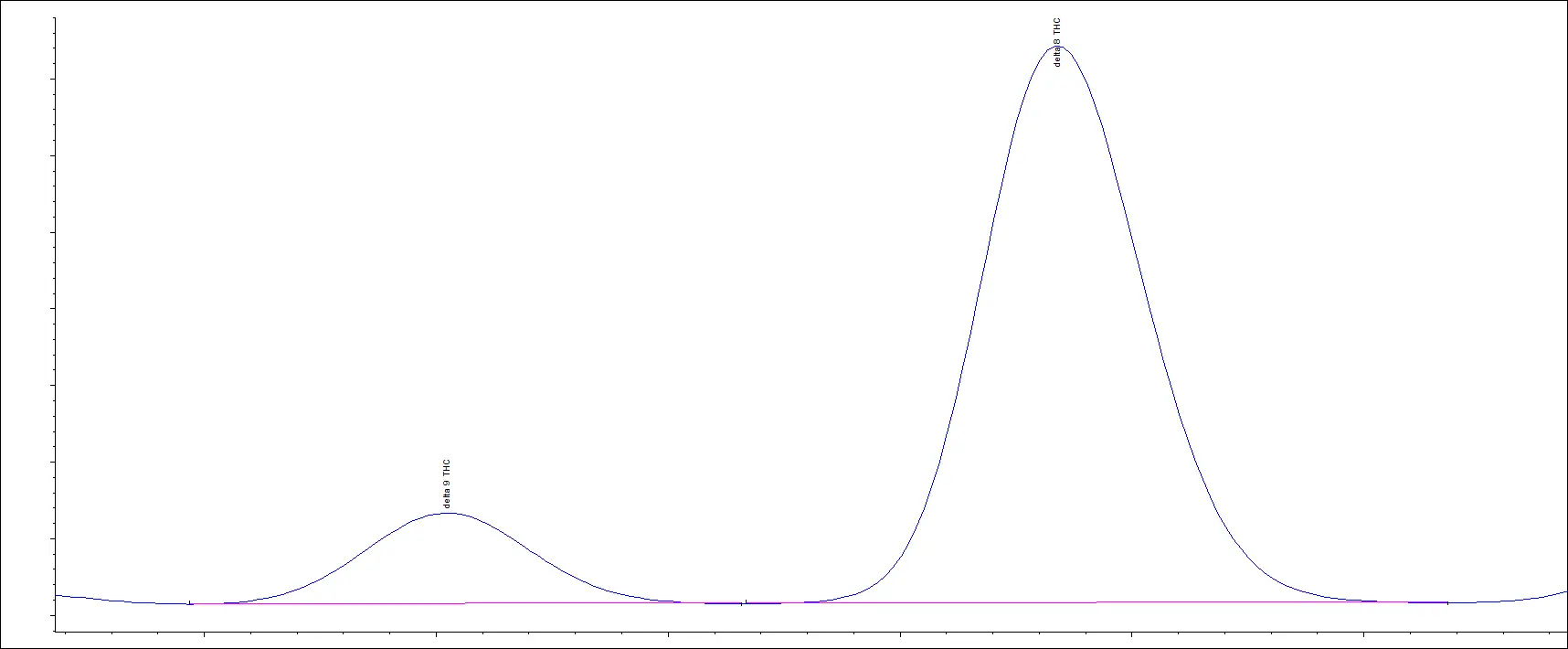
Delta-9 and Delta-8 reference standards
As a second verification we can analyze the “spectral match” for the specific compound. This is evaluating the light absorbing characteristics of each molecule against the expected characteristics. The customer sample is then compared to the library result and you can see the confirmation below.
Delta-9 THC Reference Standard (blue) vs. Customer Sample (red)
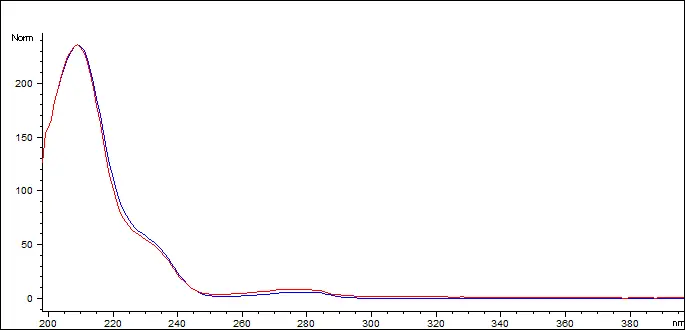
The Delta 9 THC in the sample has a spectral match of 998.616 out of 1000 (99.86%)
Delta-8 THC Reference Standard (blue) vs Customer Sample (red)
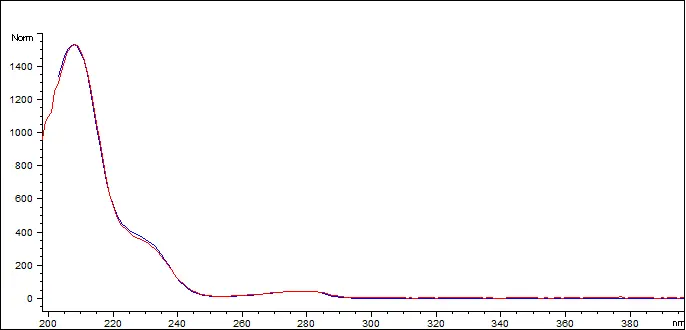
The Delta 8 THC in the sample has a spectral match of 999.596 out of 1000 (99.95%)
The process for synthesizing Delta-8 THC from CBD isolate almost always creates a certain amount of Delta-9 THC. Only hemp testing laboratories with properly developed methods, validated equipment and experienced staff can accurately determine the presence of Delta-9 THC in Delta-8 THC products. While it might seem advantageous use an inexperienced lab in the short-term, the legal and business ramifications are long lasting. What if your product DOES have Delta-9 THC and your laboratory cannot identify it? It is possible the product will be sent to a lab that can for verification.
Laboratories that are focused on defensible data can detect and quantify Delta-9 THC and Delta-8 THC, through:
- Complete separation between the chromatographic retention times of Delta-8 THC and Delta-9 THC.
- A resolution factor between Delta-8 and Delta-9 equal to or greater than the USP’s recommended 1.5 minimum.
- Secondary confirmation via UV spectral analysis.
With the rise in popularity of cannabinoids like Delta-8 THC, getting the right analytical answer for your products is imperative.
References:
[1] https://www.thermofisher.com
[2] https://www.sigmaaldrich.com
[3] https://chem.libretexts.org
